A US CTS Research Site
Pioneering Progress Through Excellence & Partnership
OUR US CTS Research Site:
OUR CLINIC:
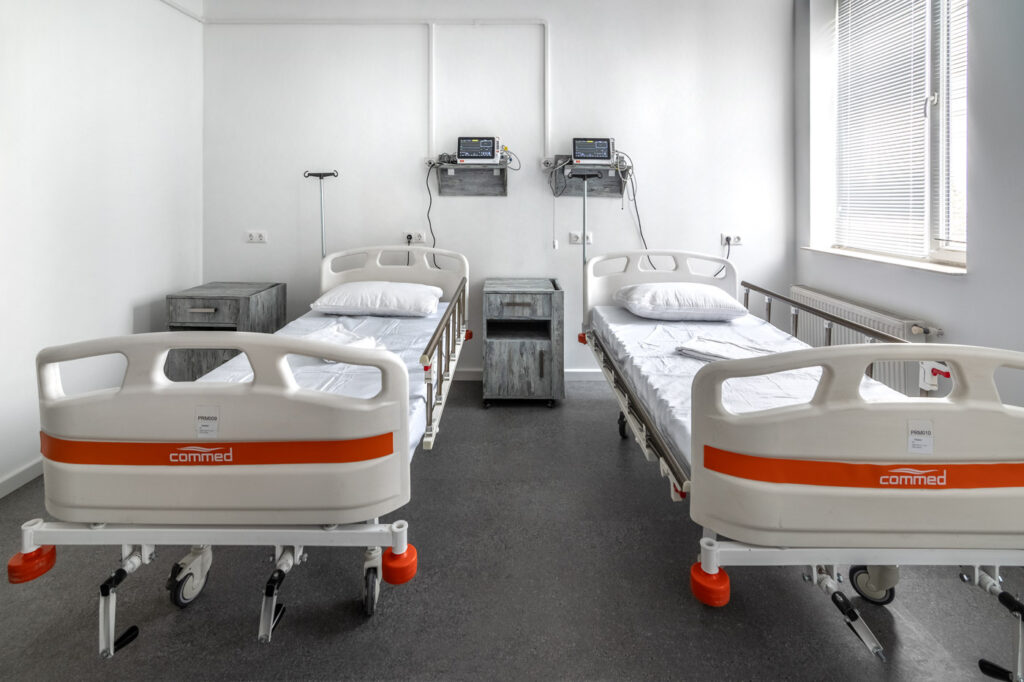
- 9 intensive monitoring beds per research clinic
- On-site, same floor ICU
- State of the Art Pharmacy per USP 797 and local regulation
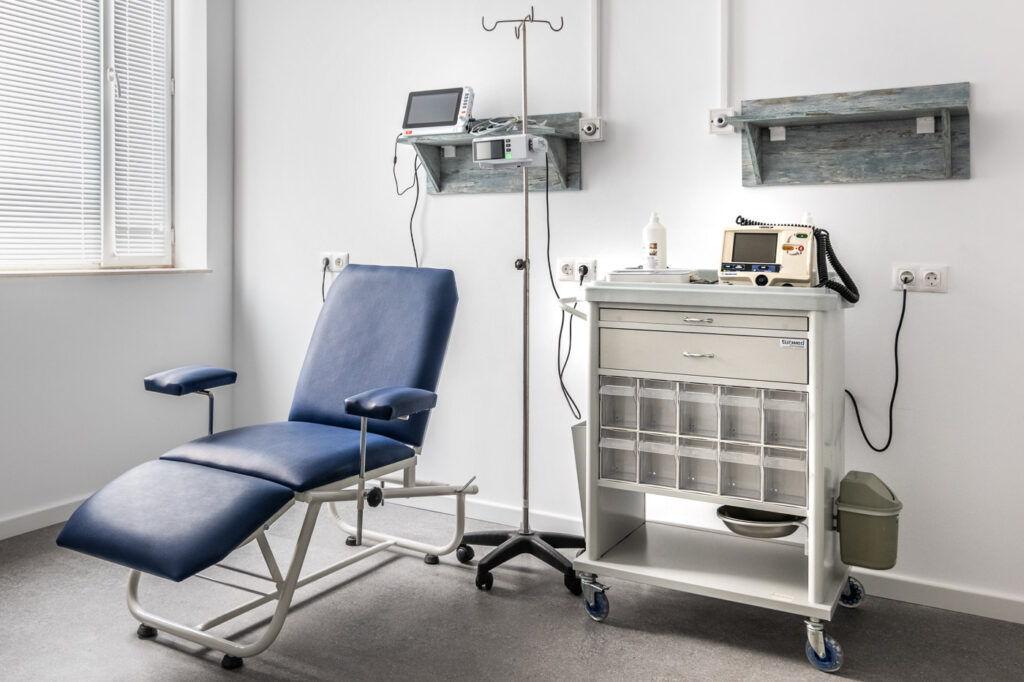
- Modern equipment: ECGs, BP monitors, centrifuges, synchronized clocks, crash cart, defibrillator, freezers -20֯C
- Monitor/CRA room
- ICF Room
- Waiting hall
We partner with investigators, nurses, technicians, pharmacists, quality assurance specialists, local project managers / site coordinators, lab specialists and regulatory affairs specialists.
Medical Director is a MD, PhD who coordinates all patient related activities from a bedside to CRF to client.
Our trials might involve sampling, sophisticated PD/biomarker collection (imaging or invasive/biopsies), and patient hospitalization or ambulatory visits.
We process and ship PK/PD/Biomarker samples to local & central labs across the world.
Patient safety is of paramount importance US CTS. All our clinics situated within the major hospitals and within seconds reach of the ICU.