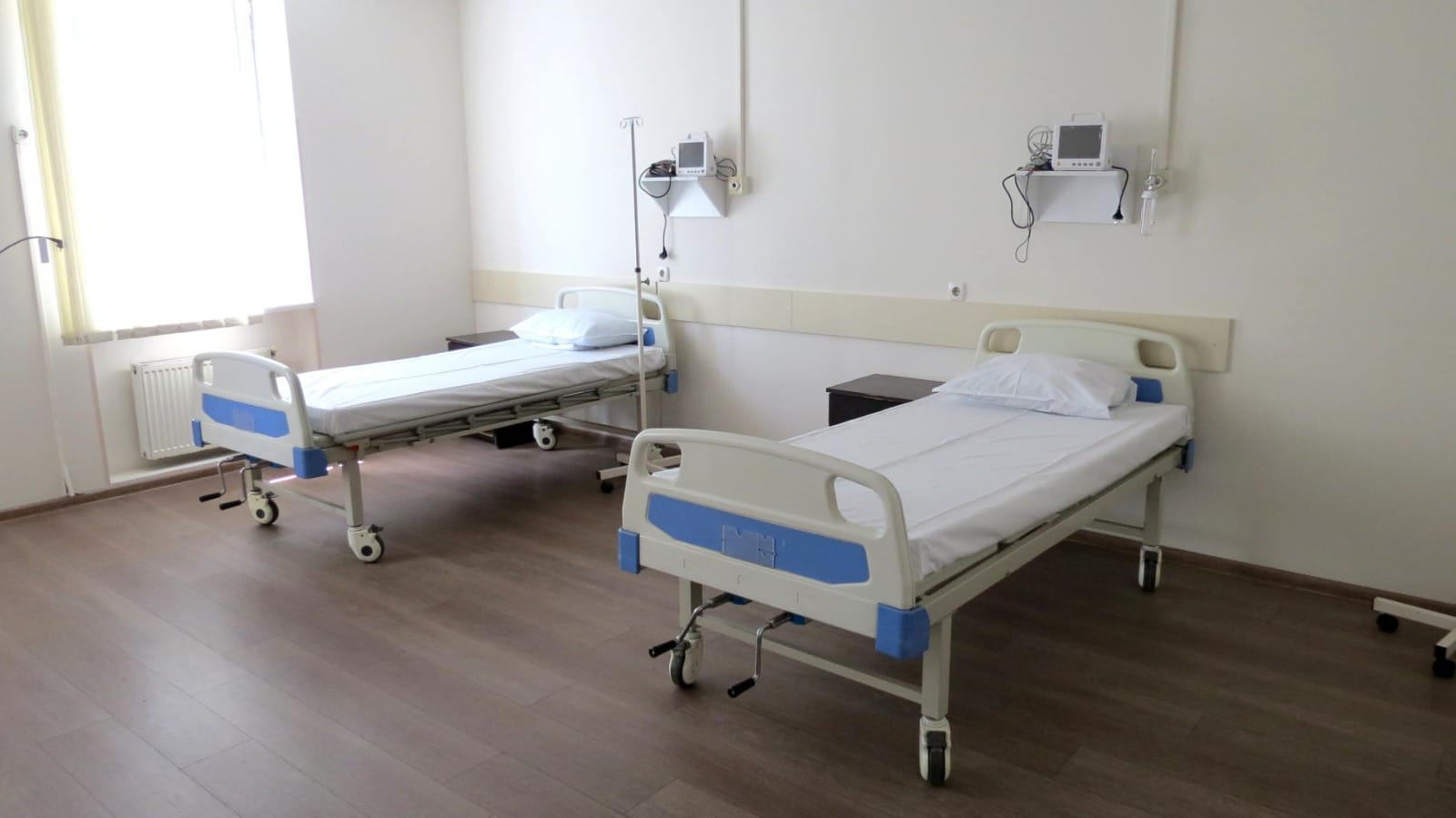
Clinical Trials in Georgia: Advancing Medical Research with Excellence
Who We Are
We are a specialized Network of Sites committed to managing Phase I/IIa clinical trials in Georgia and other countries within the former CIS (Commonwealth of Independent States). With strategically located teams in the US, UK, and Georgia, we blend global expertise with local insights to deliver high-quality clinical trial management services.
Why Georgia for Clinical Trials?
Georgia has emerged as a premier destination for clinical research due to its:
✔️ Advanced medical infrastructure
✔️ Cost-effective operations
✔️ Diverse patient population
✔️ Streamlined regulatory processes
✔️ Highly skilled medical professionals
These factors make clinical trials in Georgia an attractive option for pharmaceutical companies, biotech firms, and CROs.
📌 For more about the importance of clinical trials, visit the World Health Organization’s Clinical Trials page.
What We Do
At the heart of our operations is a commitment to conducting clinical trials in Georgia that adhere to international standards of excellence.
Our Services Include:
🔹 Regulatory Compliance: We strictly follow ICH GCP (Good Clinical Practice) guidelines and comply with major regulatory bodies such as the FDA, EMA, PMDA, and SFDA.
🔹 Site Management: From patient recruitment to data collection, we ensure that trials are conducted seamlessly and ethically.
🔹 Tailored Strategies: We develop customized solutions to address the unique requirements of each clinical study.
By partnering with us, sponsors and CROs can trust that their clinical trials in Georgia will be managed efficiently, yielding reliable and actionable results.
📌 For more on international clinical trial regulations, explore the FDA’s Clinical Trial Guidelines.
Our Team of Experts
Our team brings together over 100 years of combined global industry experience. We have collaborated with leading pharmaceutical companies, biotech innovators, and contract research organizations (CROs) worldwide, including:
✅ Pharmaceutical Leaders: Novartis, GSK, Allergan
✅ Biotech Companies: Amgen
✅ Renowned CROs: Partnerships with top-tier research organizations
📌 Learn more about the role of Contract Research Organizations (CROs) in advancing clinical research.
Top 5 Reasons to Conduct Clinical Trials in Georgia
1. Diverse Patient Population
Georgia provides access to a wide range of patient demographics, enabling researchers to gather comprehensive and representative data. The country’s genetically diverse population enhances the reliability of clinical trial results.
2. Cost-Effective Operations
Conducting clinical trials in Georgia is significantly more affordable than in Western Europe or the United States, making it a cost-efficient solution for pharmaceutical companies and biotech firms.
3. Fast Regulatory Approvals
Georgia’s streamlined regulatory framework ensures that clinical trial approvals happen rapidly, reducing delays and allowing sponsors to expedite their research timelines.
4. Modern Medical Facilities
With state-of-the-art healthcare infrastructure, Georgia offers cutting-edge medical technology and highly trained professionals for clinical research.
5. Experienced Research Workforce
Georgia has a growing community of highly skilled researchers, investigators, and medical professionals, ensuring that clinical trials are conducted with precision and compliance.
Benefits of Participating in Clinical Trials
For Participants:
✅ Access to Innovative Treatments: Patients benefit from new therapies and cutting-edge medical advancements before they become widely available.
✅ Free Medical Care: Many clinical trials offer free healthcare services, medical exams, and continuous monitoring.
✅ Contributing to Science: By joining a clinical trial, participants help advance global medical research and improve healthcare worldwide.
For Sponsors and Researchers:
🔹 Efficient Patient Recruitment: Georgia’s diverse population makes patient recruitment faster and more effective.
🔹 High-Quality Data Collection: The combination of modern facilities and experienced professionals ensures the accuracy and reliability of trial data.
🔹 Global Research Hub: Georgia serves as a bridge between Eastern Europe, Asia, and Western research communities, making it an ideal location for international clinical trials.
📌 For an in-depth look at global patient recruitment strategies, visit the Patient Recruitment Best Practices Guide.
Partner with Us for Clinical Trials in Georgia
As a trusted partner for clinical trials in Georgia, we offer unparalleled expertise in:
✅ Phase I/IIa studies
✅ Site management & patient recruitment
✅ Regulatory compliance & trial monitoring
Whether you’re a pharmaceutical company, biotech firm, or CRO, our comprehensive clinical trial services ensure the success of your research projects.
📩 Ready to collaborate? Contact us today to learn more about how we can support your clinical trial needs in Georgia and help advance medical innovation.
Frequently Asked Questions (FAQ)
1. Are clinical trials in Georgia recognized by international regulatory agencies?
Yes. Clinical trials conducted in Georgia adhere to ICH GCP guidelines and comply with the FDA, EMA, PMDA, and SFDA standards.
2. How long does it take to get regulatory approval for a clinical trial in Georgia?
Georgia’s streamlined approval process allows sponsors to receive regulatory clearance within weeks, significantly faster than in many Western countries.
3. What types of clinical trials are most commonly conducted in Georgia?
Georgia is an ideal location for Phase I/IIa studies, oncology research, infectious disease trials, and rare disease studies due to its diverse patient population and advanced medical infrastructure.
4. What are the main benefits of conducting clinical trials in Georgia?
✔️ Lower costs compared to Western countries
✔️ Fast regulatory approvals
✔️ Diverse patient recruitment
✔️ Experienced medical research teams
✔️ Modern healthcare facilities
📌 Still have questions? Contact our team for more information on clinical trials in Georgia.
Final Thoughts
With fast regulatory approvals, a diverse patient population, and modern medical infrastructure, Georgia is one of the best locations for clinical trials in Eastern Europe. If you’re looking for a cost-effective, efficient, and globally compliant solution, Georgia is the ideal choice for your next clinical study.
🚀 Start your clinical trials in Georgia today!